$129.00
At Home COVID-19 PCR Saliva Test, Active Infection
Detect a current SARS-CoV-2 infection with a non-invasive at-home spit COVID test
Find out if you, or any child over 5-years-old, has a current SARS-CoV-2 infection with 4U Health’s at-home COVID-19 saliva test. Our non-invasive PCR COVID test kit only requires an easy spit sample. No nasal swab and no tears. Simply return your sample back to the lab via prepaid FedEx Overnight shipping. You can typically expect secure hospital-grade results within 24 to 48 hours of receipt of your sample.
- Measures RNA from SARS-CoV-2 virus
- Simple at-home saliva collection
- Same lab tests offered by physicians & hospitals
- FDA authorized – recommended for ages 5+
- Kit includes free FedEx overnight return shipping to lab
- Upgrade: Screen for COVID-19, Flu, and RSV with one easy spit sample
Order now to test immediately or keep on hand for future use | Enjoy: 15% OFF
Get it for: $109.65 | Use code at checkout: COVID15
$129.00
4U Health At-Home COVID-19 PCR Saliva Test Kit
Identify an active COVID-19 infection from the privacy of your own home
Use this hospital-grade PCR saliva COVID test if you’ve recently been exposed to COVID-19, are experiencing symptoms, or need to document you don’t have an active SARS-CoV-2 infection.
- Peace of mind – PCR testing is the most accurate at home COVID test method to diagnose an active COVID-19 infection
- Accuracy – COVID saliva tests are thought to be more effective at detecting the COVID omicron variant (URL)
- Convenience – Easily collect your saliva sample without leaving home
- Painless – Avoid an uncomfortable invasive nose swab
- Confirmation – Definitively confirm a lesser grade rapid swab test
- Actionable – Your certified lab result is good for clearance (pre-op, school, work or travel)
Detects RNA from SARS-CoV-2, the virus that causes COVID-19
This saliva PCR test provides an accurate and reliable method for diagnosing COVID-19. An individual without symptoms of COVID-19 and who is not shedding SARS-CoV-2 virus would expect to have a negative (Not Detected) result in this assay. A negative result happens when the SARS-CoV-2 primers do not match the genetic material in the sample and there is no amplification. This means the sample did not contain any virus.
Easily collect a saliva sample from the comfort and privacy of home
Your COVID saliva test kit includes everything required for your easy spit sample. Spend as little as 2 minutes spitting into the self-collection device. We provide detailed instructions and a prepaid FedEx standard overnight shipping label to return your sample to the lab.
Most COVID-19 tests are done with nasopharyngeal swabs that some people describe as tickling or even stabbing their brain. Considering how invasive the typical COVID-19 swab test is, your kiddo is sure to put up a fight — and even shed some tears. Our at-home saliva COVID test, recommended for individuals 5+, is non-invasive and painless.
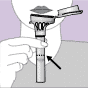
An FDA authorized test delivered directly to your door
The 4U Health COVID-19 PCR Saliva Test utilizes a saliva collection device and COVID test that have received Emergency Use Authorization from the FDA. Your kit employs the gold standard RT-PCR technology to diagnose active infections of the virus. Results are typically available within 24-48 hours after the lab receives your specimen.
Our tests bring hospital-grade results to your home
Once we receive your test, we’ll send your physician-reviewed results typically within approximately 24 to 48 hours. 4U Health at-home test reports are both accurate and easy to read. The best part is we only work with federal and state-certified laboratories. This means your results are of the highest quality, and you can share them with any clinician, travel provider, school, or employer with certainty they will be accepted.
Order COVID Tests
This Test: Only Checks for COVID-19
This Test:
Only Checks for COVID-19
COVID-19 PCR Saliva Test,
Active Infection
At-home COVID spit test to easily detect an active SARS-CoV-2 infection, whether you’re symptomatic or asymptomatic. Kit includes pre-paid FedEx return overnight shipping to lab.
- Measures RNA from SARS-CoV-2 virus
- Easy self-collection saliva sample
COVID-19 PCR Saliva Test, Active Infection
At-home COVID spit test to easily detect an active SARS-CoV-2 infection, whether you’re symptomatic or asymptomatic. Kit includes pre-paid FedEx return overnight shipping to lab.
- Measures RNA from SARS-CoV-2 virus
- Easy self-collection saliva sample
$129.00
$129.00
Upgrade Option: Screens for COVID-19 + Flu + RSV
Upgrade Option:
Screens for COVID-19 + Flu + RSV
COVID-Flu-RSV Home Test,
Saliva Collection Kit
At-home spit test to easily detect an active SARS-CoV-2, flu, and RSV infection, whether you’re symptomatic or asymptomatic. Kit includes pre-paid FedEx return overnight shipping to lab.
- Measures RNA from SARS-CoV-2, Flu A/B and RSV
- One easy self-collection saliva sample to detect all three viruses
COVID-19 Antibody Comprehensive Test
At-home spit test to easily detect an active SARS-CoV-2, flu, and RSV infection, whether you’re symptomatic or asymptomatic. Kit includes pre-paid FedEx return overnight shipping to lab.
- Measures RNA from SARS-CoV-2, Flu A/B and RSV
- One easy self-collection saliva sample to detect all three viruses
$199.00
$199.00
Out of stock
Out of stock
Digital Results
Usually within 24 to 48 hours of your sample arriving at the lab, receive secure electronic results on your device of choice

Simple
Simple to understand results designed to help you diagnose an active COVID-19 infection.
Individualized
Whether you’re symptomatic or asymptomatic, use your individualized report to confidently determine if you have COVID-19.
Useful Results
Our hospital-grade results are actionable, allowing you to use them for work or school.
How It Works

Order Your Test
Order online with express delivery. In 1 to 2 days your kit will arrive in plain packaging, ensuring a confidential testing experience.

Collect Your Sample
Your kit contains everything you need to test from home. Simply collect your saliva sample using the at-home testing kit and instructions. Then return free of charge to the lab with the provided prepaid FedEx overnight shipping label.

Fast, Accurate Results
Typically you will receive electronic results within 24 to 48 hours after receipt by the lab. Have complete trust in your lab report’s accuracy, as all 4U Health testing kits provide hospital-grade certified results.

Get Physician Support
We’ve got you covered! A licensed physician orders your test and reviews your results. When your test is positive, our clinicians may provide post-testing support to help you maximize our laboratory services.
Frequently Asked Questions
What’s included in the at-home COVID-19 PCR Saliva Test kit?
You’ll receive everything you need in order to self-collect your test specimen!
- Pre-paid shipping both ways
- Easy to follow instructions
- An at-home saliva collection kit
- Return protective envelope to FedEx Overnight your sample to the lab for testing
- Electronic passcode protected results available from your phone or computer
- Printable report to share with your doctor
- Post-test telehealth support for COVID-19 positive samples
- Help along the way if you need it
Why should I, or my child, be tested for a COVID-19 Active Infection?
Anyone ages 5+ can test for an active COVID-19 infection. This test is recommended for use with saliva samples that are self-collected by an individual age 18 years or older, or that are collected by an adult from a child 5 years of age and older. This test is intended for those who have been recently exposed to COVID-19, are experiencing symptoms, need pre-op/school/work/travel clearance or just want to test proactively to have peace of mind.
If you are experiencing one or more of the following symptoms, it increases the likelihood of having a COVID-19 infection.
- Head
- Loss of taste or smell
- Sinus congestion or runny nose
- Body
- Diarrhea
- Fatigue or general body weakens
- Fever or chills
- Muscle or body aches
- Nausea or vomiting
- Respiratory
- Cough
- Shortness of breath or difficulty breathing
- Sore throat
When would this test not be helpful?
- You should not get an At Home COVID if:
- You are experiencing severe COVID-19 symptoms. Individuals experiencing life threatening COVID-19 symptoms should seek emergency medical care right away.
- You are less than 5-years-old. The test taker must be 5+ years old.
- You are collecting your specimen in New York State. This test is not available for use in NY.
Can I use the COVID-19 At-Home Saliva PCR Test for travel purposes?
This is a FDA emergency use authorized PCR Saliva COVID test. PCR is the preferred COVID-19 testing method for many travel requirements. Many destinations require negative results within a very narrow timeframe. Included in your digital results will be a printable version that you can share. We recommend that you reach out to your destination’s government for their specific requirements and timelines before making your purchase.
While we are not able to provide an exact estimate of when you will receive your results, keep in mind the typical turnaround time for results. As a reminder:
- Order Delivery: We estimate that you will receive your order within 2-5 business days of placing your order. You also have the option of purchasing expedited shipping to your home for an additional fee.
- Returning Sample to Lab: Check with your local FedEx for pick-up and drop-off schedules. Once you ship your sample Monday-Thursday, our partner lab should receive the sample overnight by 10:30am the next business day.
- Results: Testing is batch performed Tuesday and Friday afternoons. For samples received by the lab on Monday and Tuesday, results are typically available late Tuesday afternoon. For samples received Wednesday, Thursday and Friday, results are typically available late Friday afternoon.
If you are concerned that your schedule is too tight to allow for unforeseen delays, we recommend that you check for other local testing options.
You can find the latest country-specific requirements for international travel at travel.state.gov.
What technology is used to test my saliva sample and will it identify new variants?
Our lab uses the PCR test method as recommended by the CDC. Not only is this type of test more accurate than rapid tests performed at pharmacies, it’s also able to detect all COVID-19 variants including Omicron.
Is the COVID-19 PCR Saliva Test FDA authorized?
Yep, we’ve got you covered. Both the test and the saliva collection device are FDA EUA approved.
Can I buy now and use this test later?
Test now or later. That’s great news if you are buying multiple kits for yourself or want to gift to a friend or family member for later use. All test samples must be collected and mailed by the expiration date on your kit. Our current lot of test kits expire on March 31, 2025.
Can I gift this test to a friend or family member?
All 4U Health tests are eligible for gifting. In fact, they make great presents. The recipient who receives your gift will simply open the kit, register it, and follow the collection instructions. Within a few days of sending to the lab, your friend or family member will receive secure electronic HIPAA compliant results all thanks to your generosity.
Where is my lab test performed?
4U Health tests meet national standards and are as accurate as services provided in a doctor’s office or hospital. We only work with the highest quality CLIA certified laboratories and health experts. All testing complies with state and federal regulations. And our clinicians provide medical oversight throughout the entire process.
How is my privacy protected?
Rest assured; HIPAA security standards protect your data every step of the way while determining if you have an active COVID-19 infection. Keeping your confidential data secure is our number one priority. We only share your information when required to deliver our products and services or where we are legally obligated to do so. Your results are securely protected and available for review in your online portal; always secure but easily accessible only to you.
Will my COVID-19 PCR Saliva Test be covered by insurance?
Pay upfront and receive no surprise medical bills. 4U Health is not enrolled in Medicare or any other private insurance network. This test is not eligible for Medicare or any other federal or state-funded insurance program reimbursement.
Can you provide links to technical documentation?
How may I view a sample Final Report of the COVID-19 Active Infection Self-Collection Test?
Sample COVID-19 Active Infection Report
How do I view this test’s FDA (EUA) authorization?
FDA Emergency Use Authorization: EuroRealTime SARS-CoV-2 PCR Test. Updated April 24, 2021. Accessed February 25, 2022.
FDA Emergency Use Authorization: OMNIgene Oral Collection Device. Updated October 28, 2020. Accessed February 25, 2022.
How do I view this test’s FDA fact sheet for recipients?
FDA Fact Sheet for Recipients. Updated April 22, 2021. Accessed February 25, 2022.
How do I view this test’s fact sheet for healthcare providers?
FDA Fact Sheet for Healthcare Providers. Updated April 22, 2021. Accessed February 25, 2022.
Where can I find additional information about COVID-19?
For additional information about COVID-19 antibody testing, visit the FDA website or CDC website.
Can you provide links to references and sources on the COVID-19 topic?
References and Sources
A.D.A.M. Medical Encyclopedia. COVID-19 antibody test. URL. Updated February 7, 2021. Accessed February 25, 2022.
A.D.A.M. Medical Encyclopedia. COVID-19 virus test. URL. Updated February 7, 2021. Accessed February 25, 2022.
Centers for Disease Control and Prevention. Using antibody tests for COVID-19. URL. Updated February 24, 2022. Accessed February 25, 2022.
Caliendo AM, Hanson KE. COVID-19: Diagnosis. In: Hirsch MS, ed. UpToDate. URL.
Updated January 21, 2022. Accessed February 25, 2022.
Centers for Disease Control and Prevention. Test for past infection. URL. Updated July 15, 2021. Accessed February 25, 2022.
Centers for Disease Control and Prevention. COVID-19 testing overview. URL.Updated February 24, 2022. Accessed February 25, 2022.
Centers for Disease Control and Prevention. Interim guidelines for COVID-19 antibody testing. URL. Updated January 24, 2022. Accessed February 25, 2022.
Centers for Disease Control and Prevention. Self Testing. URL. Updated February 16, 2022. Accessed February 25, 2022.
Kim AY, Gandhi RT. COVID-19: Management in hospitalized adults. In: Hirsch MS, ed. UpToDate. URL. Updated January 24, 2022. Accessed February 25, 2022.
McIntosh K. COVID-19: Epidemiology, virology, and prevention. In: Hirsch MS, ed. UpToDate. URL. Updated February 24, 2022. Accessed February 25, 2022.
The New York Times.Wondering if the vaccine worked? Get the right test, at the right time. URL. Updated June 28, 2021. Accessed February 25, 2022.
UpToDate. COVID-19: Questions and answers. URL. Updated February 21, 2022. Accessed February 25, 2022.
UpToDate. Patient education: COVID-19 overview (the basics). URL. Updated February 9, 2022. Accessed February 25, 2022.
US Food and Drug Administration. Antibody (serology) testing for COVID-19: Information for patients and consumers. URL. Updated February 24, 2022. Accessed February 25, 2022.
US Food and Drug Administration. Coronavirus (COVID-19) update: FDA issues emergency use authorization for the symbiotica COVID-19 self-collected antibody test system. URL. Updated April 6, 2021. Accessed February 25, 2022.
US Food and Drug Administration. Coronavirus disease 2019 testing basics. URL. Updated February 2, 2022. Accessed February 25, 2022.
US Food and Drug Administration. In vitro diagnostics EUAs – serology and other adaptive immune response tests for SARS-CoV-2. URL. Updated February 25, 2022. Accessed February 25, 2022.
US Food and Drug Administration. SARS-CoV-2 viral mutations: Impact on COVID-19 tests. URL. Updated December 28, 2021. Accessed February 25, 2022.
World Health Organization.Coronavirus disease (COVID-19): Serology, antibodies and Immunity. URL. Updated December 31, 2020. Accessed February 25, 2022.
Yale Medicine. Which COVID-19 test should you use. URL. Updated January 20, 2022. Accessed February 25, 2022.
Still have questions about the test?
So you still have unanswered questions. No worries, we’d love to hear from you. Reach us by e-mail, phone or chat and we will do our best to provide answers so you can determine if this is the best test for you or your child.
- 866-610-1200
- help@4uhealth.com
- Chat Support
- PO Box 100083
Pittsburgh, PA 15233



